Octane number (ON) is a measure of the detonation resistance of fuel, which is used for internal combustion engines with an external type of mixture formation. In most cases, it is used to label gasoline, and for car owners it is expressed in the usual numerical values 80, 92, 95 and 98 at gas stations. All engine ratings correspond to different engine types and the performance characteristics of cars directly depend on this.
What does octane number mean?
The octane rating indicates the chemical resistance of the fuel to fire, i.e. there is a direct relationship: the higher the OC index, the more resistant the fuel is to spontaneous combustion, the more compression it can withstand before detonation. The term itself does not reveal what the octane number is, because the standard fuel is a mixture of isooctane (not octane) and n-heptane. These 2 organic compounds were chosen as reference compounds due to their properties:
- Isooctane will not spontaneously explode even at compression ratios much higher than those created in standard gasoline engines. That is, isooctane without any impurities can be considered gasoline with an RON of 100.
- Even a small compression is enough for heptane to explode spontaneously.
Thus, OCH is a kind of scale showing the percentage ratio of the shares of isooctane and n-heptane. This definition means that gasoline, designated A-95, will have detonation properties similar to a 95% mixture of isooctane and n-heptane.
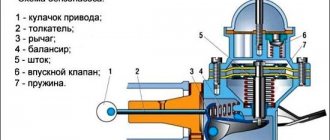
Laboratory installations for determining octane number
The octane number is determined experimentally. If it is necessary to test gasoline, it is sent for testing to laboratories that already have special engine stands.
The installation is a single-cylinder engine with carburetor power - UIT-65, UIT-85 or UIT-85M. These complexes, unlike all kinds of instruments and chemical reagents, can show a reliable octane number of gasoline.
The installations operate using a comparative method. To avoid errors, research work is carried out in accordance with GOST 8226-82 and GOST R 32339-2013. First, the engine test bench runs on a reference fuel, and operators record its characteristics. And then the results are compared with work on the gasoline under study.
Article on the topic
Fatal error? What to do if you filled up with gasoline instead of diesel
How to determine the octane number of gasoline
In most cases, the degree of OC is determined by 2 methods: exploratory (ROI) and motor (MOM). Both methods relate to laboratory methods and must comply with GOST 511/82 (for OChM) and 8226 (for OChM). The RON measurement is based on the engine operating at a lower load (compared to the RON), so its nominal value is greater than the RON. Conventionally, we can assume that the RON is the fuel characteristic when operating a car in city conditions, and the RON is on the highway.
In the Russian Federation, notations have been adopted that correspond to measurements carried out by the research method, and, for example, in America they take the arithmetic mean of the ROM and RCH. Thus, markings may vary in different countries, and this must be taken into account when traveling by personal vehicle to avoid damage to the engine.
The degree of octane is also measured independently, using special devices - octane meters, which can be found on the open market. However, it is important to understand that they cannot be used to directly determine the OC; we are talking about measuring the impedance electrical conductivity of gasoline (i.e., its dielectric constant). This parameter has some correlation with the total amount of high-octane aromatic hydrocarbons, because they stand out sharply in dielectric constant compared to other hydrocarbons contained in gasoline.
However, there is no direct functional dependence here. It turns out that one thing is being measured, but the result is something completely different. Because of this, errors are possible when, for example, when using such a device to measure the purity of gasoline of a high environmental class, containing few toxic benzene derivatives, but quite a lot of relatively harmless high-octane alkanes, the device will show a significant underestimation of the required parameter.
Express analyzers operating on the basis of an integral chemical composition are more reliable. But they must be configured for the technological processes of gasoline production, because these parameters have a significant impact on the final result. Without taking these details into account, errors may occur. In addition, such devices are expensive and not available to everyone.
Educational program. Motor gasoline, octane numbers and grades
What is octane number?
As road transport developed, it placed increasing demands not only on the quantity, but also on the quality of gasoline. Everything is clear with quantity. But what is included in the concept of quality?
Let's look at the process of gasoline combustion in an engine. This is a complex physical, chemical and technological process associated with the fulfillment of conflicting requirements. First of all, carburetion is the mixing of gasoline with air. If the fuel mixture is lean, that is, there is a lot of air and little fuel, then the combustion temperature and, consequently, the temperature of the working fluid (combustion products) in the engine decreases.
And the efficiency of any heat engine, including an internal combustion engine, depends precisely on the temperature difference of the working fluid at the beginning and end of the working process. This is an immutable requirement of thermodynamics. In addition, when operating on a lean fuel mixture, engine power decreases, the rate of coking of cylinders, pistons and valves increases, and efficiency decreases...
It is best to burn the fuel mixture with minimal excess fuel. But it is necessary to ensure uniform combustion and prevent it from being explosive.
However, not all hydrocarbons burn equally. Many of them form peroxide compounds as intermediates and their breakdown products - free radicals. All these substances are very unstable and prone to explosion. This is what happens sometimes: a spark from the flame ignites the fuel mixture, the flame front goes along the cylinder, and peroxides accumulate in its upper part. And when there is still 15-20% of unspent fuel mixture left, an explosion occurs. The speed of flame propagation increases hundreds of times - up to 2500 m/s! The shock wave is repeatedly reflected from the cylinder walls and from the piston, vibrations begin, a characteristic metallic knock appears in the engine... In a word, detonation occurs.
Under other identical conditions, n-heptane has the greatest tendency to detonation, and 2,2,4-trimethylpentane (isooctane) has the least. These hydrocarbons were taken as reference when determining the so-called octane number.
This conditional value is defined as follows. Imagine a test bench containing a single-cylinder internal combustion engine with a carburetor. During testing, this engine is started on the fuel under study, and special sensors record all mode indicators that characterize the degree of detonation. After this, a mixture of reference fuels is selected - n-heptane and isooctane, at which the engine behaves exactly the same as with the fuel under study. The detonation resistance of n-heptane is assumed to be zero, and that of isooctane to be equal to one hundred. And then it’s clear - the percentage of isooctane in the standard mixture is a characteristic of the knock resistance of gasoline. So, say, if there is 80% isooctane in the mixture, then the octane number (ON) is considered equal to eighty points.
In other words, the octane number is a relative and dimensionless quantity that has no physical meaning. But that is not all. Engines are different; The conditions in which They work are also different. For example, the stability of fuel combustion in the engine of a heavy truck operating in low gears is one thing, and detonation in a passenger car engine operating in forced mode at high speeds is quite another.
Because of this, different methods for testing the detonation resistance of gasoline have appeared in the standards of different countries. The most widespread are motor and research methods. The motor method simulates more severe engine operating conditions. In this case, the fuel mixture after carburation is heated to 149 ° C, and the rotation speed is maintained at a constant 900 rpm. According to the research method, the rotation speed is reduced to 600 rpm, and the mixture is not heated at all.
Accordingly, the octane numbers for the motor and research methods are marked differently - URO and RON.
Naturally, when using different methods, the measurement results differ, sometimes quite significantly. Thus, aromatic hydrocarbons C6-C8 give differences in the measurements of RON and URON up to 10 points.
Strictly speaking, the best picture of anti-knock resistance can be obtained from the average indicator:
(URO + RON)/2
This indicator is called the octane index. It is widespread in American specialized literature. However, it has not yet been adopted as an official standard. To evaluate different grades of commercial gasoline, one index is usually selected. So, according to GOST; The octane number of motor gasolines A-66, A-72 and A-76 is measured using the motor method. But high-octane gasolines AI-93, AI-95, AI-98 are tested using a research method, as indicated by the letter “I” in the gasoline brand.
Let me tell you, it has become clearer, but not by much, so below I am posting more material about octane numbers and clippings from a reference book on motor gasoline.
Octane numbers
How are research, engine and compression octane numbers determined? In the early 20th century, engineers were puzzled by the behavior of airplane engines that would self-destruct for no apparent reason. Suddenly, while the engine was running, a hole formed in its pistons. They soon found out that the culprit was a fuel explosion, and the problem was found in the changing quality of the fuel. It became obvious that a fuel rating system was needed.
At the time, the batches of fuel that were measured appeared to be identical, but there was still a large difference in quality, even among batches that originated from the same factory.
Therefore, fuel manufacturers began to try to compare fuel quality using a series of chemical tests, which proved unreliable in determining when a given fuel would explode or detonate when used in the real world of engines.
Therefore, special single-cylinder, variable-compression engines were created as a standardized testing platform. Now all you had to do was compress the fuel being tested with the crank, right up to the point of detonation, and then record - this will be the highest possible compression ratio of gasoline. Such engines were distributed to various fuel laboratories and a measurement standard was born.
Or so the testers thought.
But after testing in different places, it was discovered that the same fuel shows different compression ratio numbers depending on atmospheric conditions. It was then decided to select two pure and readily available substances to calibrate all test mechanisms. In order for chemically pure substances to give predictable, constant performance at which it would be possible to set a standard for the “highest” and the “lowest” level.
Again, two primary standard fuels, isooctane (2,2,4-trimethylpentane) and n-heptane, were randomly selected and assigned "octane" numbers of 100 and zero, respectively. Then, all test engines could be "zeroed" using n-heptane, while the upper range could also be determined using isooctane (2,2,4-trimethylpentane).
By the way, octane turned out to be an insufficient name from the point of view of “assessing knock resistance”, because on the n-octane molecule (C8H18) it actually leaves -17!
The "octane" rating of a fuel is based on a comparison of the knock characteristics of various mixtures of n-heptane and isooctane. For example, a 92 octane rating means that the fuel being tested, under normal operating conditions in a standard engine, performs as well as a mixture that consists of 92 parts isooctane and eight parts n-heptane. Numbers above 100 simply indicate the potential to perform better than pure isooctane.
But since those early days, the fuel standardization procedure has been divided into several types of tests, the most common of which are research octane number, engine octane number and compression octane number.
Research octane number and engine octane number tests use the same single-cylinder variable compression engine, but differ from the compression octane number test, which is determined at higher engine intake speeds.
Next, an excerpt from the automobile reference book about gasolines and their brands.
Motor gasoline
To prepare motor gasoline, straight distilled gasoline, thermal cracking gasoline, catalytic cracking and catalytic reforming gasoline, coking gasoline (for low octane gasoline), alkyl gasoline, isopentane, toluene (for high octane gasoline), butane, butane-butylene fraction, pentane-amylene fraction are used. and gas gasoline. To increase the knock resistance of motor gasoline, anti-knock additives are used, the most common of which are tetraethyl lead (TEP) and methyl tert-butyl ether (MTBE). Used as a fuel for spark-ignition internal combustion engines.
The sulfur content in motor gasoline varies from 0.05 to 0.1%. Motor gasoline is produced in winter and summer grades, which differ in saturated vapor pressure. Marked by octane numbers measured using the motor or research method, or both methods simultaneously.
They produce motor gasoline of the brands A-72 (leaded and unleaded, summer and winter), A-76 (leaded and unleaded, summer and winter), A-80 (leaded and unleaded), AI-91 (unleaded, summer and winter), A-92 (leaded and unleaded, summer and winter), AI-93 (leaded, summer and winter), AI-95 “Extra” (unleaded summer) and AI-95 (unleaded, summer and winter).
Different regions of the world use different brands of motor gasoline. In Europe, the most common brands are “superplus” or “super” (unleaded, summer and winter), “premium” or “European” (unleaded, summer and winter), “German” (leaded, summer and winter), “Italian” (leaded , summer and winter), “regular” (unleaded, summer and winter). In the USA, motor gasoline of the following brands is used: regular, midgrade, premium and superpremium. All brands come in both summer and winter versions. In the USA, only unleaded or, rather, low-leaded motor gasoline with a lead content of less than 0.0026 g/l is used. In the Asia-Pacific region, motor gasoline of the brands 91RON, 92RON, 95RON, 97RON is used. All of them are low-leaded (summer) with a lead content of up to 0.01 g/l. The abbreviation RON is made up of the first letters of the English words research octane number (research octane number). When writing gasoline brands, different octane numbers are used, so when selecting analogues, you need to read the description of each brand. The production of motor gasoline in the world is approximately 900 million tons per year, which is 30% of the total production of petroleum products.
Motor gasoline grade A-72 Low octane motor gasoline
Low quality motor unleaded gasoline with a lead content of no more than 0.013 g/l. Contains products of thermal and catalytic cracking, coking and pyrolysis, straight-run gasoline and antioxidant additives. Density is not standardized. The octane number according to the motor method is 72; according to the research method, it is not standardized.
Motor gasoline grade A-76 Low octane motor gasoline
Low quality motor gasoline. Contains products of thermal and catalytic cracking, coking and pyrolysis, straight-run gasoline, antioxidant and anti-knock additives. The most common brand of gasoline for use in agriculture.
A-76 is produced leaded (yellow) with a lead content of no more than 0.17 g/l and unleaded (colorless) with a lead content of no more than 0.013 g/l. Density is not standardized. The octane number according to the motor method is 76, and according to the research method it is not standardized, but is usually close to 80. When determining the export price of gasoline of this brand, the base grade is Naphta with a discount of 10-12 US dollars per 1 ton.
Motor gasoline A-80 Low octane motor gasoline
Regular quality motor gasoline. Contains anti-knock additives. produce leaded with a lead content of no more than 0.15 g/l and unleaded with a lead content of no more than 0.013 g/l. Sulfur content - no more than 0.05%. Density - no more than 0.755 g/cmA-803. The octane number according to the motor method is 76, and according to the research method - 80. In fact, this is a brand of gasoline with slightly improved characteristics.
Motor gasoline brand A-92 Regular motor gasoline
Regular quality motor gasoline. Contains anti-knock additives. The most common brand of gasoline in large cities of the Russian Federation and Ukraine. produce leaded with a lead content of no more than 0.15 g/l and unleaded with a lead content of no more than 0.013 g/l. Sulfur content - no more than 0.05%. Density - no more than 0.77 g/cmA-923. The octane number according to the motor method is 83, and according to the research method - 92. The quality is close to the European brand “Regular” and the Asian brand 92RON, but contains 30% more lead.
Motor gasoline AI-91 AI-91 regular motor gasoline
Regular quality motor gasoline. Contains anti-knock additives. produce unleaded (colorless) with a lead content of no more than 0.013 g/l. Sulfur content - no more than 0.1%. Density is not standardized. The octane number according to the motor method is 82.5, and according to the research method - 91. The quality is close to the European brand “regular” and the Asian brand 91RON, but contains 30% more lead.
Motor gasoline AI-93 AI-93 regular motor gasoline
Regular quality motor gasoline. Leaded AI-93 is prepared on the basis of mild catalytic reforming gasoline, with the addition of toluene and alkyl gasoline. To increase the vapor pressure, a direct distillation fraction with a boiling point of up to 62°C or a butane-butylene fraction is added. Unleaded AI-93 is prepared on the basis of severe catalytic reforming gasoline with the addition of alkyl gasoline, isopentane and butane-butylene fraction. Contains anti-knock additives.
AI-93 is produced leaded (orange-red) with a lead content of no more than 0.37 g/l and unleaded (colorless) with a lead content of no more than 0.013 g/l. Sulfur content - no more than 0.1%. Density is not standardized. The octane number according to the motor method is 85, and according to the research method - 93. Leaded AI-93 was produced especially for export without adding dye, with a lead content of no more than 0.15 g/l and sulfur no more than 0.001%. When determining the export price of gasoline of this brand, the base grade is the European “regular”.
Motor gasoline AI-95 AI-95 premium motor gasoline Motor gasoline of improved quality. Prepared on the basis of catalytic cracking gasoline of light distillate feedstock with isoparaffin and aromatic components and the addition of gas gasoline. Contains anti-knock additives. produce unleaded (colorless) with a lead content of no more than 0.013 g/l. Density is not standardized. The octane number according to the motor method is 85, and according to the research method - 95. The quality is close to the European premium brand and the Asian 95RON, but contains 30% more lead.
Motor gasoline AI-95 “Extra” AI-95 Extra premium motor gasoline
Improved quality motor gasoline. Prepared on the basis of catalytic cracking gasoline of light distillate feedstock with isoparaffin and aromatic components and the addition of gas gasoline. Contains anti-knock additives.
AI-95 is produced unleaded (colorless), there is no lead in it. Density - no more than 0.720 g/cm3, sulfur content - no more than 0.05%, saturated vapor pressure - no less than 53.3 kPa (400 mm Hg). The octane number according to the motor method is 85, and according to the research method - 95. The quality is close to the European premium brand and the Asian 95RON, but better, since it does not contain lead.
www.auto-most.ru
Compression ratio and RH
Compliance with the compression ratio OCH is regulated by various GOSTs. The corresponding values can be found in the table.
| Gasoline brand | EYES | OHM | Compression ratio |
| A-72 | — | 72 | 7 |
| A-76 | — | 76 | 7.5 |
| AI-80 | 80 | 76 | 8 |
| AI-91 | 91 | 82.5 | 9 |
| AI-92 | 92 | 83 | 9.2 |
| AI-93 | 93 | 85 | 9.3 |
| AI-95 | 95 | 85 | 9.5 |
| AI-96 | 96 | 85 | 9.6 |
| AI-98 | 98 | 87 | 10 |
The effect of gasoline with an octane rating higher or lower than that recommended by the manufacturer on the engine
If a car whose engine is designed for 95 or 98 gasoline is filled with low-purity fuel, you will soon notice uncharacteristic noises. Engine performance will decrease, consumption of the fuel mixture will increase, and heat will begin to flow into the exhaust catalyst, which will reduce its strength. If such a situation occurs, you should slowly, monitoring the speed, drive to the nearest gas station and fill in the required brand of gasoline.
Many car owners know about the dangers of low-octane brands and try to avoid them. However, there is another danger, which is the misconception that high-octane fuel is always better, that it is simply better gasoline. However, it is not.
An operating value that is too high compared to the recommended value will cause overheating of the seats and exhaust valve disc. The valves will quickly burn out and will require lengthy engine repairs at a high cost. Similar to the previous situation, fuel consumption will increase, efficiency will begin to decrease, and carbon deposits will appear on the pistons, valves and spark plug electrodes.
To avoid all these problems, only fill the fuel tank with gasoline recommended by the manufacturer.
What fuel is considered high octane?
Once upon a time, car enthusiasts boasted to each other about a full tank of 95, swearing that they would never again drop to 92, much less to 80, since with a new brand of gasoline the car literally flutters, the engine roars in a new way, and in general, transport travels “softer” along the highway. However, now even the 95th will not surprise anyone, because it has been replaced by the AI-98 and AI-100.
Gas stations also have “premium” brands of 95 and 98 in their arsenal, which are sold at a special price that differs from the supposedly less productive source by literally a couple of rubles.
As for the value of octane numbers, we are talking only about the resistance of a particular brand of gasoline to detonation - the combustion process of the fuel-air mixture.
A simple example of a stove with dry and wet wood will help you understand this process more clearly, because it is quite logical that the latter will burn much worse than the former.
Combustion of gasoline with different octane numbers
The combustion rate of gasoline is directly related to the octane value. High octane fuel is characterized by a smooth and long combustion time. At the same time, the engine runs more smoothly and evenly compared to engines designed for low-octane fuel. And vice versa, the lower the octane rating, the shorter the period required for ignition of the fuel-air mixture. This affects fuel consumption, because... faster combustion also means faster flow of gasoline into the combustion chamber.
However, it must be taken into account that savings cannot be achieved by simply increasing the OP, because You still need to take into account the design features of the engines.
What is the detrimental effect of detonation on an engine?
Knock resistance is the ability of a fuel to ignite and then burn in the cylinders of a car engine, bypassing unwanted explosive processes. Although the detonation combustion of gasoline-air mixtures will not completely destroy the engine, like a TNT block, it should still be taken into account that during detonation the flame front can propagate at a speed of approximately 2000 m/s (instead of the required 20-40 m/s), which can be compared with the action of the classical explosives.
All this leads to ringing knocking sounds, the consequences of which can be destructive for the engine: the oil film on the walls of the liner is torn off by hypersonic shock waves, increasing wear on the piston rings and cylinders. The smoke of the exhaust increases, the engine begins to overheat, its power decreases, and the combustion chamber and piston surface are destroyed. As a result, all these factors reduce the normal life of the engine.
Increasing and decreasing the octane number of gasoline
Gasoline, which is obtained from oil by simple distillation, is called straight-run. This grade is characterized by a low OR in the range from 41 to 56. This fuel is not suitable for modern engines, therefore reforming techniques, as well as catalytic and thermal cracking, are used in industrial conditions to increase the OR to accepted standards.
But sometimes everyday situations arise when it is necessary to increase or decrease the octane number. For example, modern gas stations almost no longer sell fuel grades 76/80, but equipment designed for low-octane gasoline is still available, so there is a need for such fuel.
The opposite situation may also arise when the engine is designed for high-octane gasoline 95/98, but the gas station only has 92 grade gasoline. Modern chemistry comes to the rescue, which will allow you to save the engine by independently increasing the engine rating with the help of special substances - universal type additives and otan-correctors.
Portable devices for determining octane number
At the moment, this method of determining octane number is the most reliable. Of course, not every driver can use it in practice, because the UIT-65 and UIT-85 motor units cannot be carried in a pocket or mounted in a garage.
However, now there are portable devices for express fuel analysis on sale that can determine the octane number based on indirect signs. These are OKTIS or Digatron devices. They measure the dielectric constant of gasoline by the amount of anti-knock additives. If there are few additives, then the liquid’s resistance to electric current differs from the picture shown by fuel with a large number of anti-knock additives.
Take two containers, one is filled with fuel tested for UIT-65 or UIT-85, and the other is filled with the fuel being tested. The device does not show the exact octane number, but the difference between fuels.
The measurement error of instruments varies within 2-3 units. They cannot be used on an industrial scale.
The most common additives
To convert gasoline into low-octane gasoline, the addition of kerosene is most often used. The disadvantage of this method is the difficulty of selecting the required proportions. Despite the imperfection of household measuring instruments, to increase accuracy it is recommended to determine the OC of the resulting mixture using octanometers.
To increase the octane rating of modern gasoline, special additives are used, which are also called anti-knock agents, consisting of alcohols and ethers, the maximum octane value of which can reach 120. Examples of the most common additives are:
- Octane Plus from the American manufacturer Hi Gear is capable of increasing engine rating by 5-6 units, helping to reduce fuel consumption and wear of valves and piston rings.
- Octane Plus from the popular German manufacturer Liqui Moly - increases HP from 2 to 5.5 units. An important factor is the grade of gasoline used: for the same volume of fuel, the grade 92 grade will increase more than the grade 95 gasoline.
- Russian-made Totek UMT, in addition to increasing engine efficiency, helps to increase engine efficiency, reduce fuel consumption, remove moisture in the fuel system, and also protects the working surface of valves and injectors from carbon deposits, etc.
In addition to these additives, car owners are offered a large number of other products with different characteristics, but it should be borne in mind that all additives are characterized by high volatility, so modern fuel with a high content of them is not suitable for long-term storage.